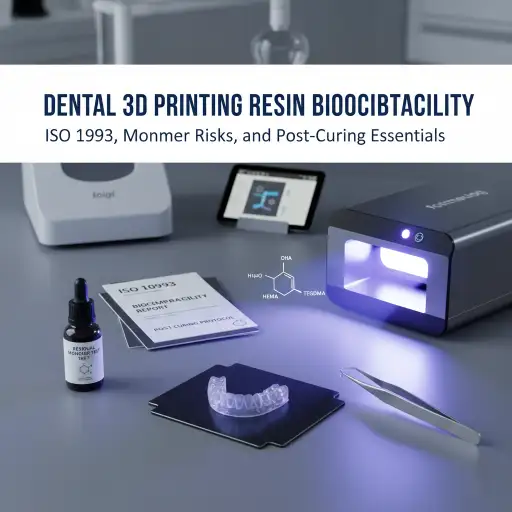
The shift to 3D printing has brought speed and precision to dental workflows, creating appliances from surgical guides to temporary crowns. However, the convenience of digital manufacturing must never overshadow the single most important factor: Biocompatibility. A dental resin is only viable if it is proven *not* to cause adverse effects—such as allergic reactions, inflammation, or toxicity—when in contact with a patient’s oral tissues. For every dental professional, verifying this safety is crucial for clinical success and regulatory compliance.
1. Decoding the Standards: Your Compliance Checklist
To guarantee patient safety, dental 3D printing resins must meet stringent global standards. These regulations provide the technical requirements and testing methods that every professional must understand.
A. ISO 10993: The Global Medical Device Foundation
This international standard series is the core requirement for proving biological safety. When evaluating a material, look for documented compliance with the following tests:
- Cytotoxicity (ISO 10993-5): This test determines if the material’s leachable substances kill or damage cells. A passing score typically requires cell viability to be 70% or greater. Failure to pass this test indicates a toxic material.
- Sensitization and Irritation (ISO 10993-10): This assesses the risk of the material causing a local reaction or a full-body allergic reaction.
- Chemical Characterization (ISO 10993-18): This step uses advanced analytical techniques to identify and quantify every component that could potentially leach out, including unreacted monomers or heavy metals.
B. ISO 7405: The Specific Dental Requirement
This standard works in parallel with ISO 10993 but focuses specifically on the unique needs of dental medical devices (implants, restorations, appliances) that contact oral tissues. It dictates the appropriate selection of testing methods for the oral environment, ensuring that the evaluation is clinically relevant.
C. Market Compliance: FDA and CE Mark
For a resin to be legally used in the market, it must have:
- FDA Approval (U.S.): For most intraoral resins, this requires 510(k) clearance based on comprehensive data proving compliance with ISO 10993.
- CE Mark (Europe): Under the Medical Device Regulation (MDR), the resin must be audited by a Notified Body, demonstrating full compliance with ISO standards and a robust quality management system (e.g., ISO 13485).
2. The Hidden Threat: Residual Monomers and Their Toxicity
The biggest safety challenge in photopolymer (light-cured) 3D printing resins is the presence of residual monomers. These are small chemical components that do not fully link together during the printing process.
- The Risk: These unreacted chemical components can leach out from the final printed part and enter the patient’s mouth, leading to adverse effects.
- Common Toxic Agents:
- HEMA (Hydroxyethyl Methacrylate): A known culprit that can induce oxidative stress in dental pulp and gingival cells, potentially causing inflammation and cell death.
- TEGDMA (Triethylene Glycol Dimethacrylate): This monomer is associated with DNA damage and cell cycle disruption. Its toxicity is often magnified when combined with other residual monomers.
The takeaway for clinics and labs: If the polymerization is incomplete, you are exposing the patient to potentially toxic chemicals.
3. The Solution: Post-Curing as the Safety Guardian
The post-curing (secondary curing) process is not merely a step for achieving final mechanical properties; it is the most critical factor for ensuring biocompatibility.
Mechanism of Action:
- Promoting Polymerization: Post-curing utilizes controlled light (e.g., 405 nm) and often heat (50 to 75 degrees Celsius) to drive the final chemical reaction within the part.
- Reducing Leachable Components: This energy encourages the remaining unreacted monomers to form additional chemical bonds, which locks them into the material structure.
- The Result: Proper post-curing can reduce residual monomers by 75% or more, transforming a cytotoxic material into a safe, biocompatible medical device.
Practical Tip: Always strictly adhere to the manufacturer’s specific instructions regarding post-curing time, temperature, and equipment specifications. Deviating from the protocol is a direct compromise of the product’s safety rating.
4. Procurement Excellence: A Safety-First Purchasing Strategy
For those responsible for purchasing dental materials, your selection process is the front line of patient safety.
- Verify Testing Reports: Do not accept a simple claim of “biocompatible.” Demand to see the formal test reports proving compliance with ISO 10993 and ISO 7405.
- Audit Post-Curing Protocols: Ensure that the resin’s required post-curing process is feasible and reliable within your lab or clinic’s existing workflow and equipment.
- Focus on Consistency: Choose suppliers known for rigorous quality control and technical support. Inconsistent resin batches can lead to unpredictable safety outcomes.
By prioritizing transparent compliance, understanding the risks of residual monomers, and strictly implementing the post-curing protocol, dental professionals can fully harness the benefits of 3D printing while upholding the highest standards of patient care.